EY refers to the global organization, and may refer to one or more, of the member firms of Ernst & Young Global Limited, each of which is a separate legal entity. Ernst & Young Global Limited, a UK company limited by guarantee, does not provide services to clients.
Pharmaceutical Revenue Management Services
With our knowledge and experience, we can bring a powerful blend of technical experience, industry insight and robust automation tools to the table that can help drug manufacturers meet complex government pricing and Medicaid rebate requirements.
Drug manufacturers participating in government drug pricing and rebate programs need to fully understand the complex set of nuanced regulations, as well as capture, understand and analyze complicated data sets and inputs to fulfill the myriad federal and state program and contract obligations. Interpreting those rules accurately and correctly can mitigate compliance risks, and sometimes make the difference between profit and loss on a new drug program.
Through our cutting-edge technology and team of resources with deep industry and regulatory knowledge, we provide a full range of support aimed at helping you improve efficiency and meet your obligations.
- By providing a more comprehensive view of your data, we help you focus on your proprietary advantages and identify new product strategies.
Optimize your go-to-market strategy
- Obtain a better ROI in government pricing contracts by mining your data for key insights. We model multiple scenarios so you can avoid limiting your revenue potential for one-off deals.
Reduce revenue leakage
- Securing a more comprehensive view of your obligations to the government helps only pay what you owe.
Experience
- Class of trade automation
- Product launch
- Government pricing restatement/Managed service
- Savings through Medicaid processing
As a part of a Medicaid Drug Rebate program pricing restatement effort, we worked with a major pharmaceutical manufacturer to reclassify more than 300k customer class of trade (COT) assignments using several data sources and a proprietary algorithm. We assigned >95% of customers through automation, saving significant time and money for our client. Since implementing the automation for ongoing support, manual review hours have been reduced, enabling our client to focus on operational efficiency and analytics.
We assisted a biopharmaceutical manufacturer that was in the midst of a product launch enter contracts with the Department of Veterans Affairs (VA) and Centers for Medicaid and Medicare Services (CMS) for participation in the Federal Supply Schedule and Medicaid Drug Rebate programs. We supported the client in drafting methodology and policy documents, gathering data needed for calculations (using EY technology) and considering pricing implications associated with participation in government pricing programs. This supported a smooth and efficient product launch.
We guided a major pharmaceutical manufacturer through a large Medicaid Drug Rebate program pricing restatement. This project spanned multiple years, involving updates to product master attributes, customer classes of trade and transaction type classifications to comply with CMS rules and regulations. Following the successful restatement, the client determined that the EY solution was more efficient and customized to their needs and transitioned to our solution for ongoing government pricing operations, calculations and Medicaid processing.
Using the EY Medicaid Rebate processing technology, our clients have been able to recuperate significant and material amounts. When a manufacturer does a government pricing restatement or best price (BP) true-up that results in a decreased unit rebate amount (URA), many states do not automatically submit adjustments for overpayments. Our technology automates the identification of those situations so the manufacturer can apply those balances toward rebates owed to states, preventing revenue leakage.
EY can help
Whether you outsource your entire operation to us, or just work with us to analyze your data, we can help you benefit from our knowledge and experience with government pricing contracts as well as with multiple life sciences companies.
Our teams can help manage the process efficiently and consistently, making the process easier and less complicated so your people can focus on what’s really important: driving value for your organization through analytics and strategic insights.
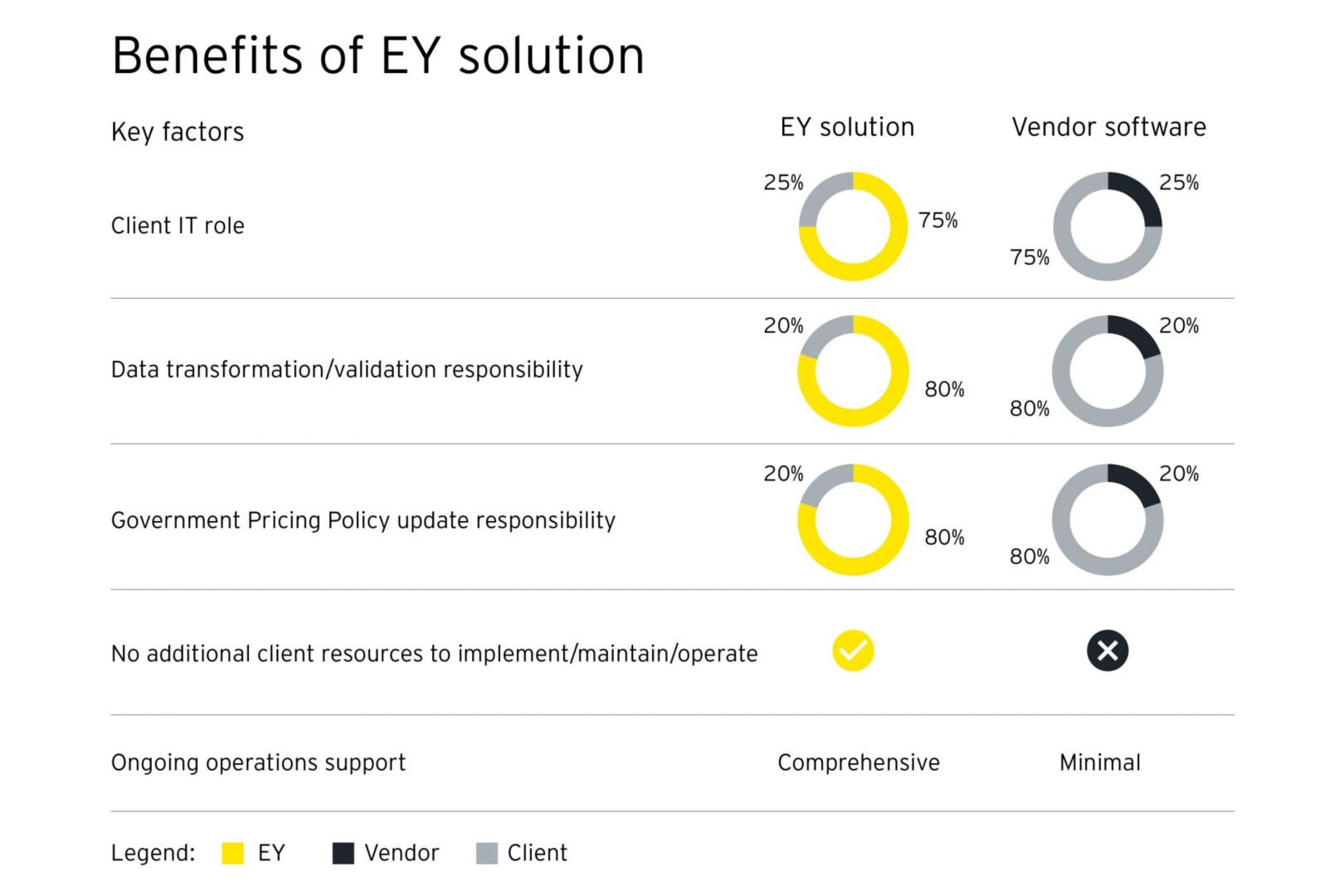
We can compare options with your current software vendor, and explore where EY can add value and efficiencies across the board.
The team
Alixe Bonelli
A principal with Ernst & Young LLP. Provides prospective, reactive and managed compliance-oriented services to life sciences entities participating in government contract programs.
Sajeev Malaveetil
Enabling businesses and communities to serve our government and the people they represent — ethically, honestly and with integrity.
Eliza Biedziak
Forever curious, practical and efficient. Proactively seeking process improvement to drive innovation.
Garrett Pape
Experience in government pharmaceutical pricing programs and related compliance services for the pharmaceutical industry and health care providers.
Our latest thinking
MDRP proposed rule could impact 340B covered entities
340B covered entities should be aware of four changes proposed by the Centers for Medicare and Medicaid Services. Read to learn more.
How CMS proposes to compensate 340B covered entities for underpayments
A CMS proposed rule will compensate covered entities affected by the Medicare reimbursement cuts for 340B drugs. Learn more.
Beyond HRSA: 340B risks related to Medicare and Medicaid
Are 340B entities giving enough attention to non-HRSA compliance obligations? Learn more.
How US government pricing programs affect drug product launches
Before a drug product launch, it is important for organizations to analyze how government programs can influence their products. Learn more.
How CMS Medicaid Drug Rebate Program final rule could impact companies
CMS issues Medicaid Drug Rebate Program final rule. Learn more.