By integrating global trial data, scientific publications and partnership signals, a deal radar platform that continuously scans these dynamic regions to uncover high-potential opportunities can surface from emerging innovation hubs and empower deal teams to act early on licensing opportunities.
Artificial intelligence can be the key to modernizing the search and evaluation process, creating an always-on deal radar that can sort through these signals – including academic literature, scientific papers and competitive intelligence reports to find the best candidates.
Growing role of agentic AI in continuous biopharma deal landscape surveillance
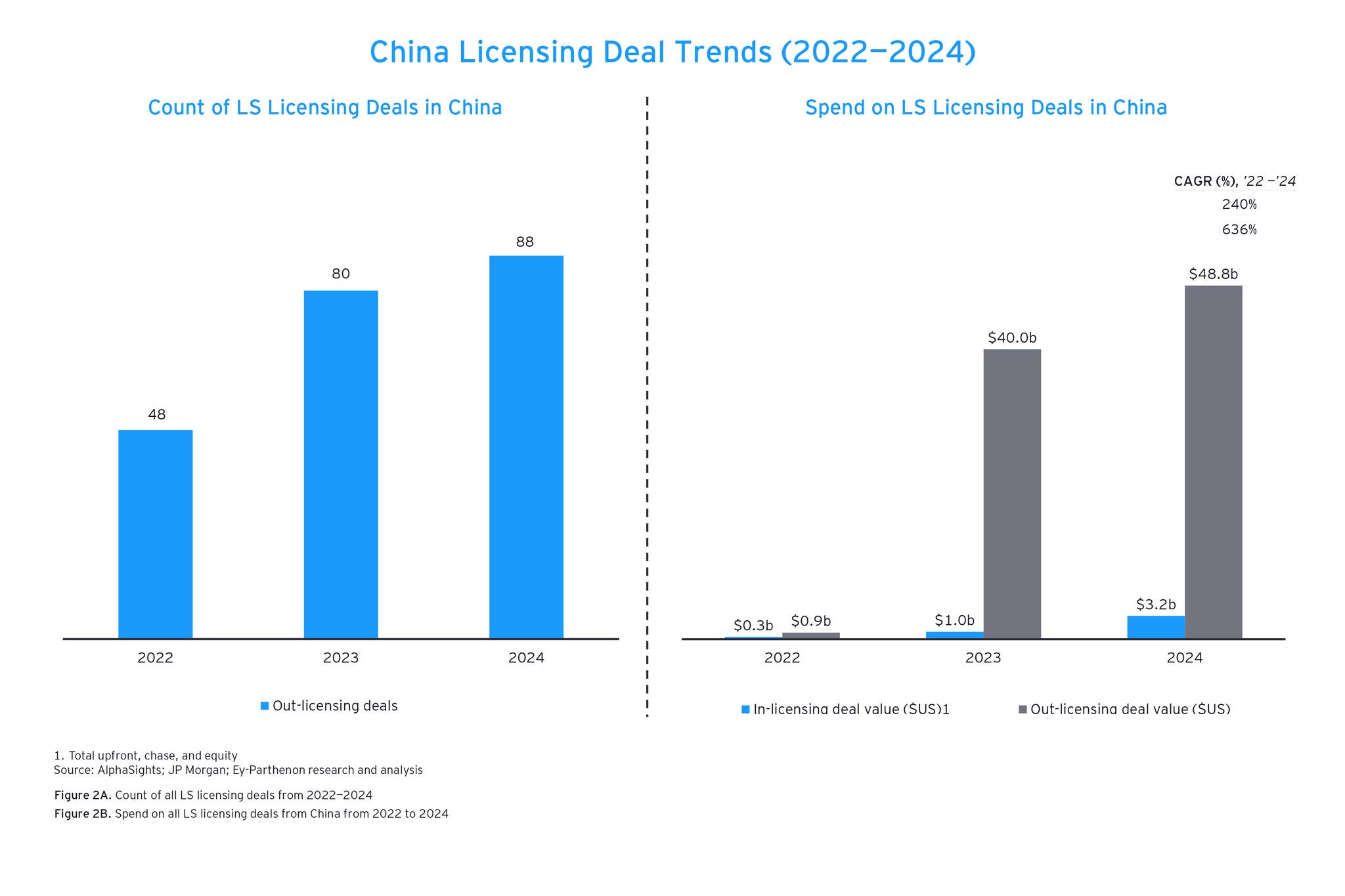
The current search and evaluation process has flaws that can hinder the ability to identify and acquire promising deal candidates earlier or identify warning signs that a therapy might not be successful. Current approaches often rely on sporadic literature reviews or conference attendance for these signals, which can cause delays.
Meanwhile, deal teams need to monitor siloed databases and generating analysis from these databases is highly labor-intensive. Not only is data siloed, but the workflows and tools used by teams are disjointed, meaning it can take even more time to gather and analyze the data.
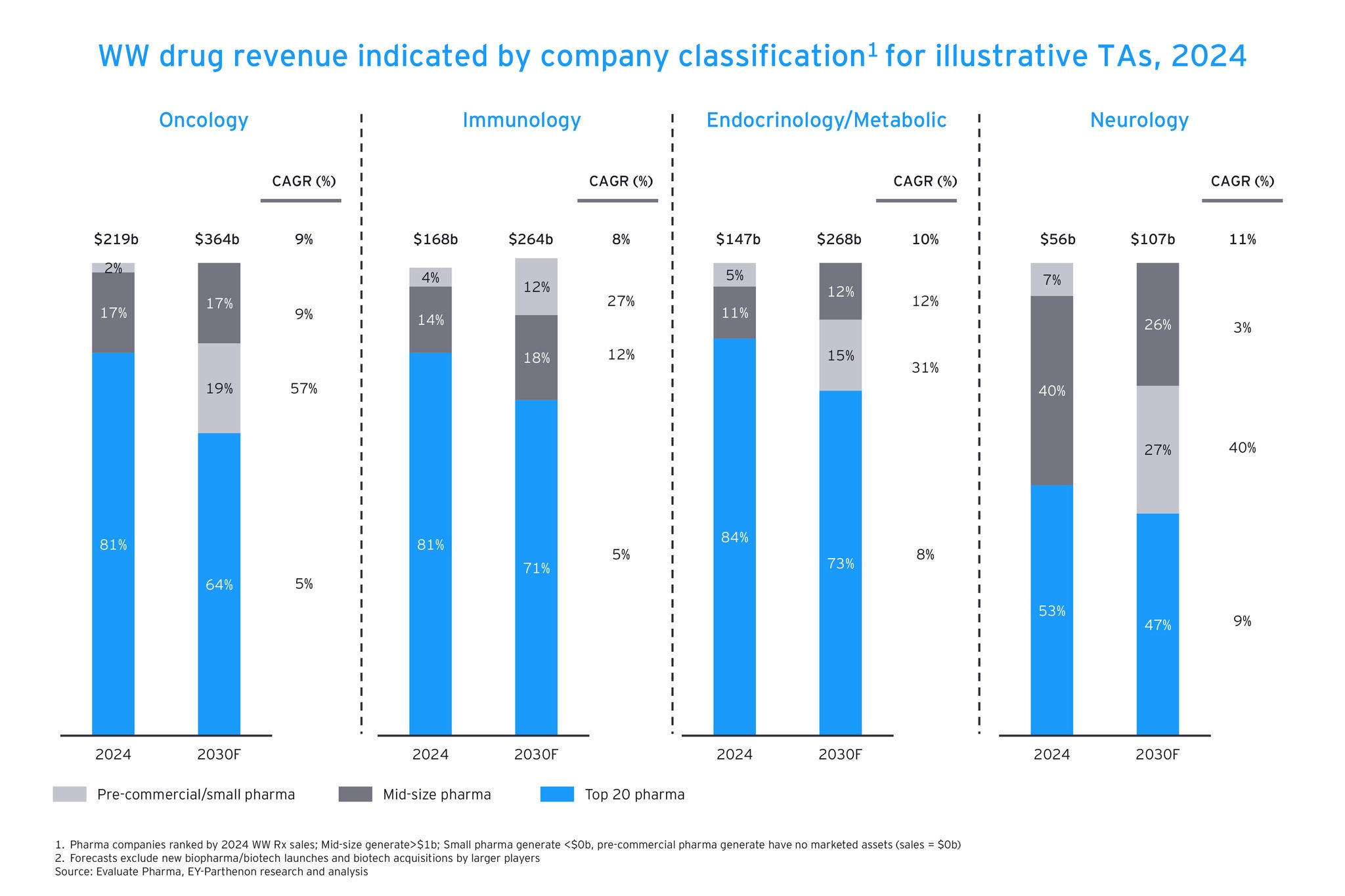
Deal teams want a comprehensive profile of each prospective target – scientific merit, clinical trial results, patents, regulatory status, commercial potential, competitive positioning and even team and talent strength — and they want it at a granular level. They need to know the landscape for the potential treatment, including patient needs, which competitors have similar programs and where the whitespace that can be exploited lies. They also need this in real time, with constant updates rather than sporadic, outdated snapshots.
They also need to know how a potential target will fit within the company’s wider portfolio and to have comprehensive deal benchmarking to inform valuation and deal structuring.
Recent advances in artificial intelligence (AI), especially in natural language processing and autonomous agents, offer a breakthrough opportunity to overcome these challenges. For biopharma M&A and licensing, a new generation of AI-powered solutions is being developed to help identify and evaluate targets more quickly and effectively.
Think of these as AI deal radars that continuously read scientific papers, tracks clinical trial registries, monitors news feeds, query databases and even generate summaries or draft analyses – all tailored to a biopharma’s strategic interests.
The AI agents that power the deal radar
This “always on” deal radar is driven by a series of AI agents that can augment human intelligence rather than replacing it.