EY refers to the global organization, and may refer to one or more, of the member firms of Ernst & Young Global Limited, each of which is a separate legal entity. Ernst & Young Global Limited, a UK company limited by guarantee, does not provide services to clients.
EY Clinical Trial Fast Lane
The EY Clinical Trial Fast Lane solution accelerates clinical trial site activation to bring novel therapies to patients faster while reducing costs and resource needs for sponsors and sites.
Your business challenge
Clinical trial site activation timelines are a leading indicator of overall clinical trial performance, yet they remain a leading cause of delays in clinical development. Long trial startup timelines can delay or prevent patients from accessing novel therapies, exacerbate challenges in clinical trial enrollment and retention, and present significant opportunity costs for industry sponsors and clinical trial sites alike.
Solution benefits
The EY Clinical Trial Fast Lane solution accelerates high-priority clinical trials, delivering up to a tenfold reduction in site activation timelines, which enables sponsors and sites to:
- Execute trials faster to solidify progress against strategic, operational and financial goals
- Deepen strategic partnerships by prioritizing key sponsor-site relationships through differentiated activation timelines
- Improve engagement and personnel satisfaction through improved, streamlined site activation processes
Case study: Accelerated clinical trial activation at UCSD MCC | EY - US
Solution features and functionality
Our EY Clinical Trial Fast Lane concierge and functional support team members bring deep knowledge of clinical trial site operations, integrating directly into site activation functions to drive progress excellence and execute key study startup milestones.
Clinical trial sites that use our solution receive hands-on support to optimize and accelerate key site activation milestones, such as:
- Clinical trial agreement development and negotiation
- Coverage analysis
- Clinical trial budget development and negotiation
- Regulatory reviews, including submissions and follow-ups to institutional review boards (IRBs) or other safety committees
- Study calendar builds and/or treatment plan builds
- Coordination of site trainings, system access and the site initiation visit
EY Clinical Trial Fast Lane has delivered significant reductions in US site activation timelines:
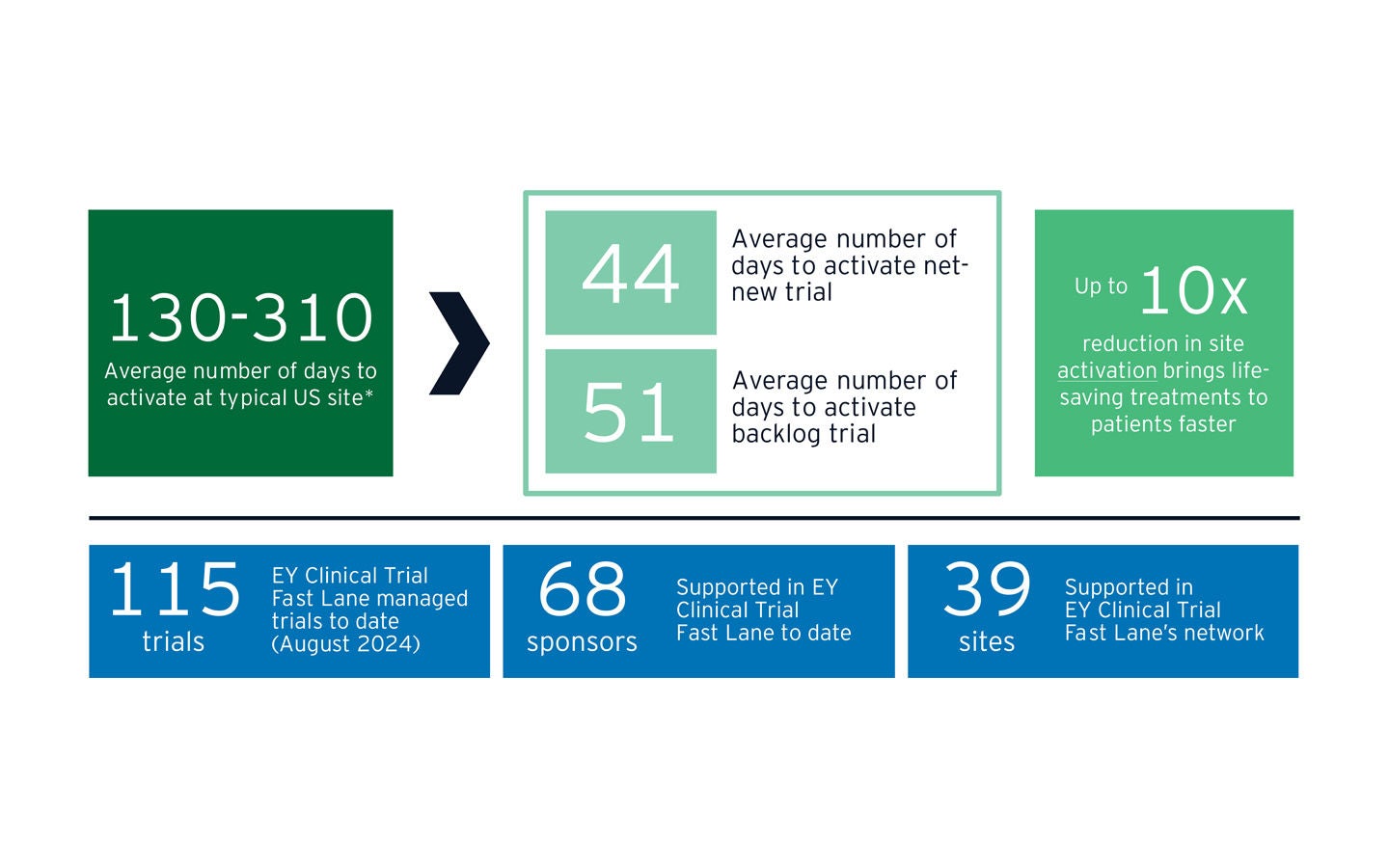
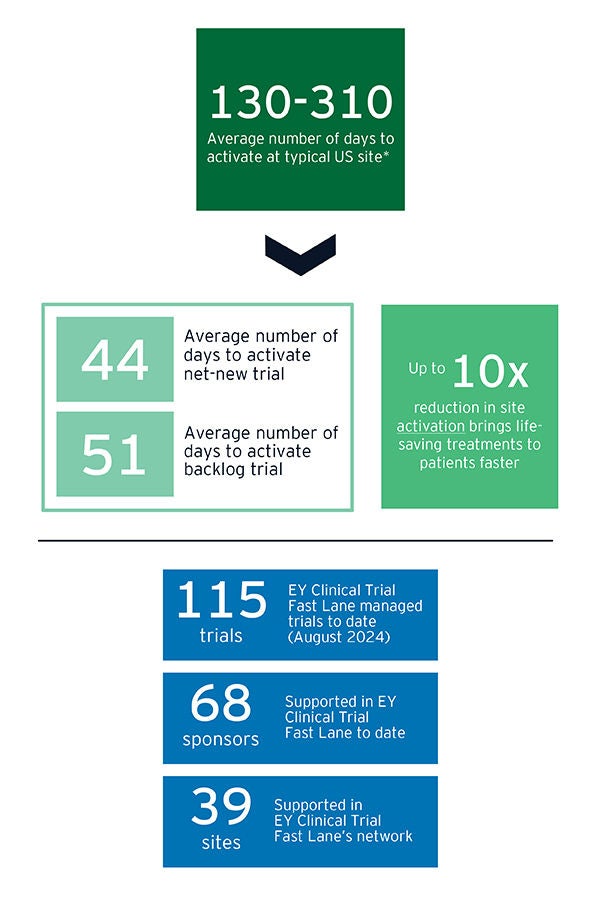
* Net-new trials measured from completion of site feasibility review through to site activation. EY Clinical Trial Fast Lane involved from start. Days are counted as working days. Backlog trials measured from start of EY Clinical Trial Fast Lane site engagement through to site activation. Activation “struggling” prior to EY engagement. Majority of EY Clinical Trial Fast Lane sites are US academic medical centers and/or NCI-designated (comprehensive) cancer centers.
Why EY
We accelerate clinical trial activation, leveraging our strong experience and understanding of clinical trial operations at the intersection of the health and life sciences sectors and across the R&D continuum.
FAQs
How EY can help
-
EY Studio+ helps organizations identify, define and design products and services that deliver long-term value. Read more on studio.ey.com.
Read more -
EY Data and Insight-Driven Transformation teams can help you use technology and data to modernize and innovate your business and prepare for future disruption. Learn more.
Read more -
EY Business reinvention consulting services can help your business deliver long-term value for customers, employees, businesses and society. Learn more.
Read more



