EY refers to the global organization, and may refer to one or more, of the member firms of Ernst & Young Global Limited, each of which is a separate legal entity. Ernst & Young Global Limited, a UK company limited by guarantee, does not provide services to clients.
How EY can help
-
Optimize operations with Ernst & Young LLP Supply Chain consulting services. Use tech and AI to boost resilience, efficiency and value.
Read more
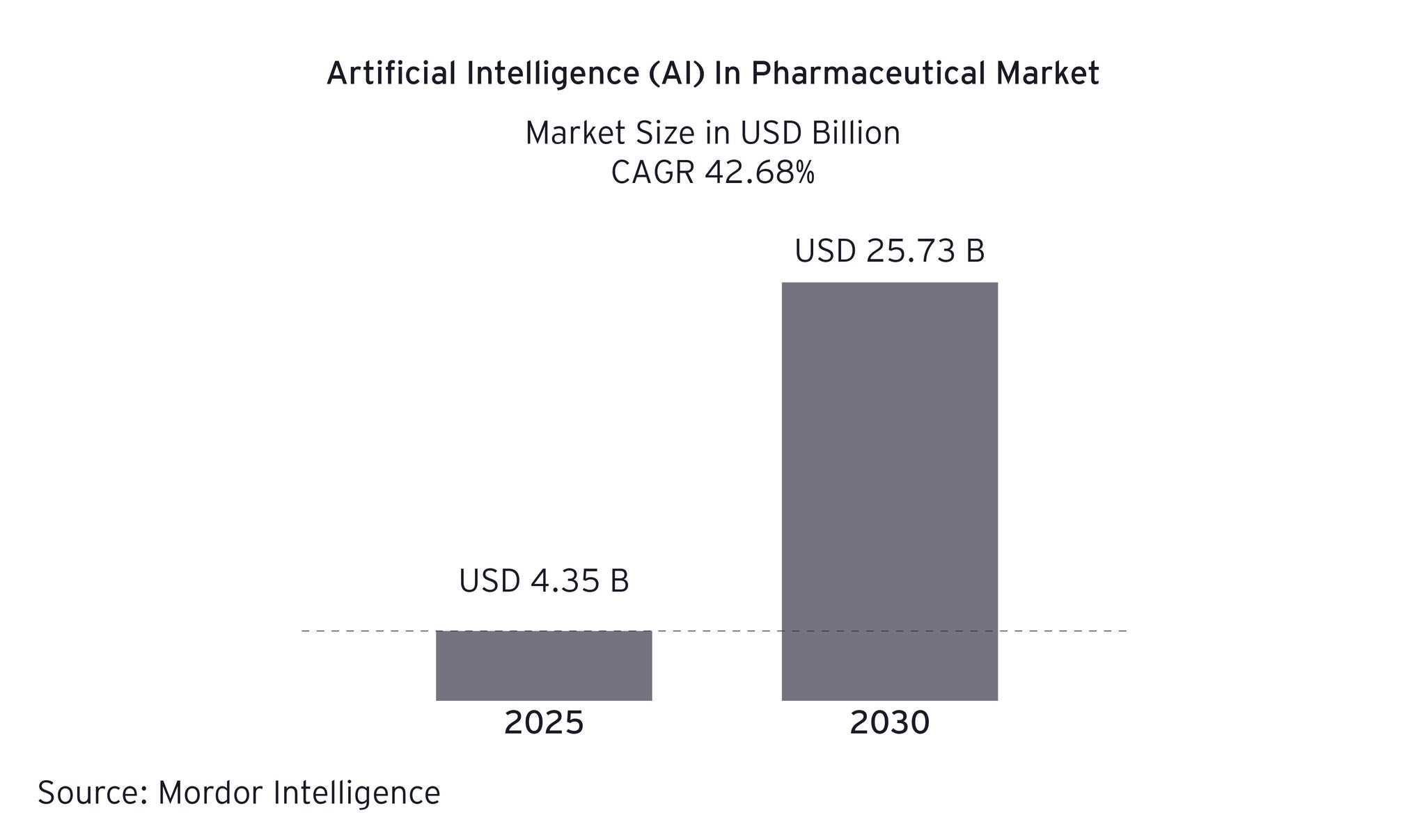
The five-layer data mesh solution in pharmaceutical manufacturing
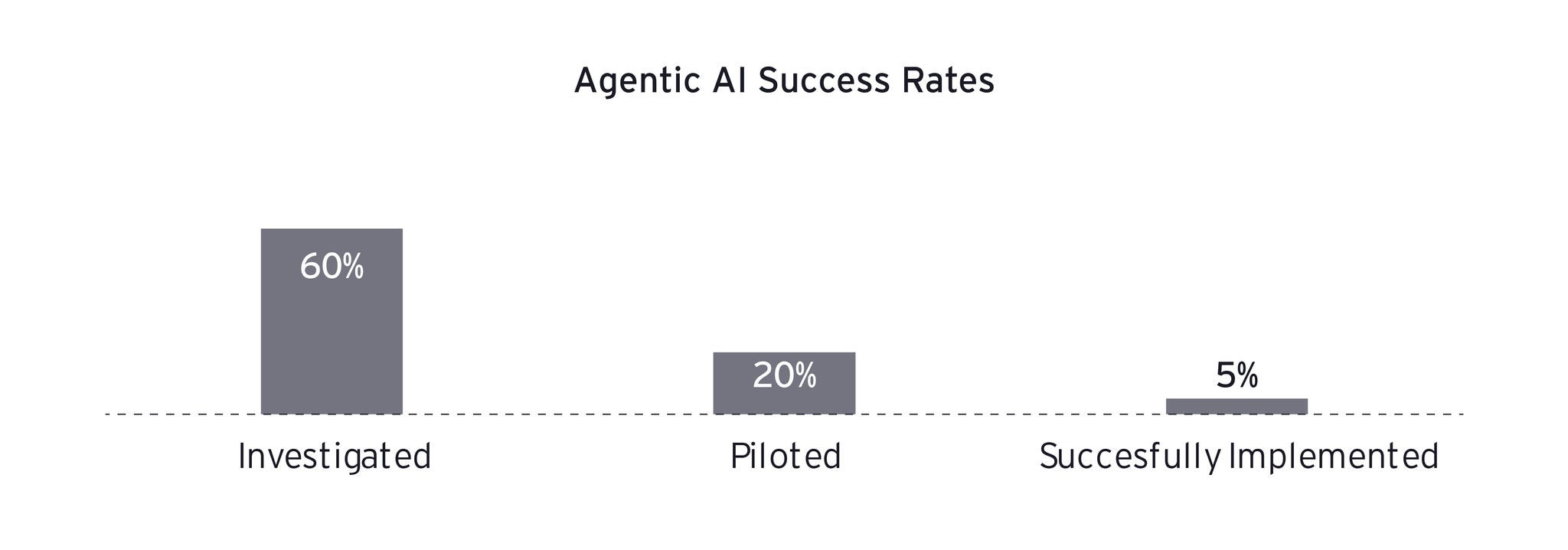
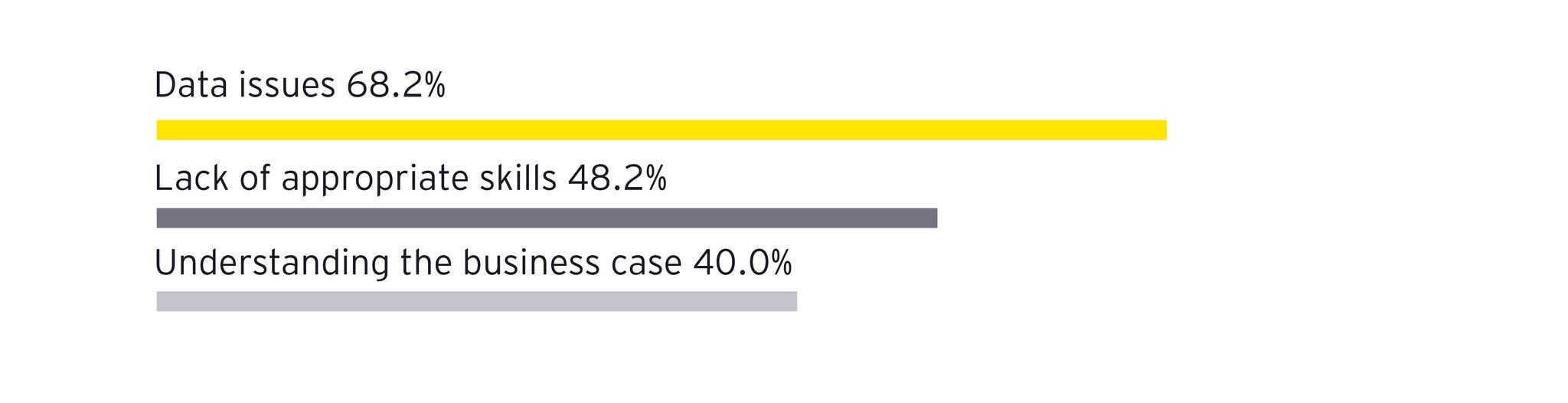
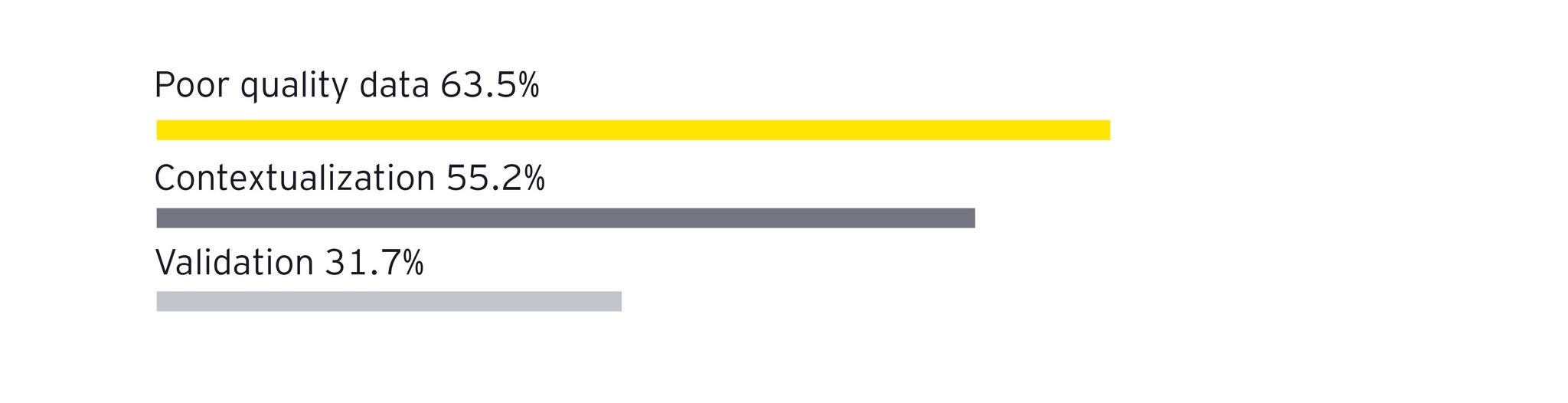
To overcome these challenges, pharmaceutical manufacturers can adopt a five-layer data mesh strategy that transforms chaotic data into AI-ready information without disrupting existing operations. This approach treats data as a product and fosters domain-driven ownership and federated governance.
Layer 1: Edge intelligence (the foundation)
Instead of collecting uncontextualized data, organizations should tag data points with relevant context at the moment of creation. This principle emphasizes decentralized data ownership, ensuring that domain experts (manufacturing operators) enrich data at its source.
Implementation strategy:
- Deploy small, cost-efficient edge gateways machine by machine.
- Include machine ID, batch number, process phase and calibrated time stamp at data collection.
- Use Network Time Protocol (NTP) for time synchronization, which is available in most networks.
Layer 2: Unified name space (single source of truth)
When an organization establishes a unified name space, every signal has a standardized name and location. This eliminates the need to sift through multiple systems for specific data elements.
Implementation strategy:
- Publish data to organized topics: plant, area, line, machine and measurement.
- Define naming conventions for production lines and begin publishing.
- Create self-service data discovery through standardized interfaces.
Layer 3: Common data models (universal language)
Standardizing definitions for key data elements fosters consistency across all plants and systems, creating a framework for interoperability.
Implementation strategy:
- Develop schema definitions for five to 10 core objects in pilot use cases.
- Maintain a simple registry for storing and versioning schemas.
- Validate all data against registered schemas before acceptance.
Layer 4: Federated governance (standardization and tracking without central control)
This layer emphasizes the need for comprehensive tracking of data, regardless of its location, while allowing for local implementation of standards. In the pharmaceutical context, federated governance is particularly crucial because of the industry’s stringent regulatory requirements and the need for compliance with Good Manufacturing Practices (GxP).
By adopting federated governance, pharmaceutical manufacturers can maintain data quality and compliance across all operations, fostering a culture of accountability and continuous improvement.
Implementation strategy:
- Establish central governance bodies that set organization-wide compliance standards, which local teams then adapt to their specific processes and regional requirements.
- Maintain audit trails to satisfy regulatory requirements while enabling innovation and flexibility in operations.
- Implement domain-specific risk controls aligned with manufacturing criticality levels so that each site can address its unique challenges while adhering to overarching governance principles.